Original
Hidrogeles de gelatina entrecruzados para aplicaciones en Ingeniería Tisular. Un estudio preliminar
Gelatin-crosslinked hydrogels for Tissue Engineering applications. A preliminar Study
Actual. Med. 2018; 103: (803): 9-12 DOI: 10.15568/am.2018.803.or02
Enviado: 26-02-2018
Revisado: 01-03-2018
Aceptado: 28-03-2018
RESUMEN
Los biomateriales juegan un papel esencial en el desarrollo de nuevos sustitutos tisulares ya que proporcionan el entorno tridimensional esencial para promover la adhesión, migración y proliferación celular. En este sentido, los biomateriales naturales aportan moléculas biológicamente activas que típicamente promueven la adhesión y el crecimiento óptimo de las células. La gelatina es un biomaterial cuyo alto contenido de colágeno y cuya red tridimensional interconectada podría contribuir a la generación de tejidos artificiales similares a los tejidos nativos. En este contexto, diferentes técnicas de entrecruzamiento químico han sido utilizadas con el fin de mejorar las propiedades físicas y mecánicas de los biomateriales de uso en Ingeniería Tisular. El glutaraldehído (GA) es un entrecruzante químico bien conocido que puede proporcionar materiales con una mejora sustancial en las propiedades de tracción. El objetivo de este artículo es determinar la concentración óptima de GA en hidrogeles de gelatina para determinar la posible aplicación de estos nuevos biomateriales entrecruzados en la clínica traslacional. A este respecto, se obtuvieron resultados interesantes que podrían ser útiles para diseñar andamios con propiedades controladas según su grado de entrecruzamiento que facilitaría la producción de productos más similares a los tejidos nativos. Los hidrogeles de gelatina entrecruzados con GA al 5% mostraron patrones morfológicos adecuados que sugieren una posible aplicación para la regeneración del tejido cardiovascular.
Palabras clave: gelatina, ingeniería tisular, entrecruzamiento, glutaraldehído.
ABSTRACT
Biomaterials play a key role in the development of new tissue substitutes as they provide the essential 3D environment to promote cell adhesion, migration and proliferation. In this sense natural biomaterials offer biologically active molecules which typically promote excellent cells adhesion and growth. Gelatin is a biomaterial whose high collagen content and its interbranching 3D network could certainly contribute to the construction of more native-like tissues. In this context, different techniques of chemical cross-linking have been used in order to improve the physical and mechanical properties of biomaterials for use in Tissue Engineering. Glutaraldehyde (GA) is a well-known chemical crosslinker that can provide materials with substantial improvement in tensile properties. The aim of this article is to test different GA concentrations to crosslink gelatin hydrogels to evaluate the potential application of these new crosslinked biomaterials according to the specific properties of the different tissues in the translational clinic. In this regard interesting findings were obtained that could be helpful to design controlled-properties scaffolds regarding its crosslinked degree that would facilitate the production of more suitable tissue-like products. The proposed 5% GA crosslinked gelatin hydrogels shown morphological patterns and meet the requirements of a first macroscopic and microscopic evaluation which suggests a potential application for the regeneration of cardiovascular tissue.
Keywords: gelatin, tissue engineering, crosslinking, glutaraldehyde.
Leer Artículo Completo
INTRODUCTION
Tissue engineering is a multidisciplinary and interdisciplinary field involving the development of bioartificial tissues and organs with the purpose of repairing or enhancing tissue or organ function, the resulting bioartificial constructs consist of cells, biomaterials and trophic factors (1).
Biomaterials are used to generate the scaffold which will provide the appropriate 3D environment to promote cell adhesion, migration and proliferation (2). Moreover, the scaffold must provide adequate form and structural support for the intended anatomic site (3). Scaffold materials for tissue engineering can be broadly classified as either synthetic or naturally occurring in origin. Synthetic biomaterials are manufactured with a tailored architecture, and their degradation characteristics controlled by varying the polymer itself or the composition of the individual polymer including polystyrene, poly-l-lactic acid (PLLA), polyglycolic acid (PGA) and poly-dl-lactic-co-glycolic acid (PLGA) (4). However, its main drawbacks are its reduced biocompatibility and bioactivity. On the other hand, natural materials including polymers such as gelatins, silk and chitosan are biologically active molecules and typically promote excellent cell adhesion and growth (3).
Gelatin is a heterogeneous mixture of peptides derived from the parent protein collagen by procedures involving the destruction of cross-linkages between the polypeptide chains along with some breakage of polypeptide bonds (5). Two types of gelatin are generally obtainable, depending on the pre-treatment procedure (prior to extraction process). Acidic pre-treatment (type A) does little affect the amide groups while the alkaline pre-treatment (type B) targets the amide groups converting many of the asparagine and glutamine residues to aspartate and glutamate (6). According to the literature, both collagen and gelatin used as scaffold components improve significantly infiltration, adhesion, spreading, and proliferation of cells on resulting scaffolds. Introduction of natural biopolymer has beneficial effect on biological recognition signals and thus cells are expected to migrate deeper into the scaffold. Additionally, improved elasticity and deformability facilitate formation of new or expansion of existing cavities for cell penetration (7).
Despite the known advantages and wide applicability of biomaterials, there are several limitations that restrict their use for biomedical applications (8). Mainly, they lack adequate mechanical properties and in many instances the stability in aqueous and physiological environments required for medical applications (9). In this milieu, crosslinking techniques has been broadly used to overcome the limitations of biomaterials (10). According to this, glutaraldehyde (GA) is a well-known chemical crosslinker that can react with functional groups in both proteins and carbohydrates, and can provide materials with substantial improvement in tensile properties (11).
The aim of this study is to evaluate different GA concentrations to crosslink type A gelatin in order to evaluate the robust potential of modified gelatin in tissue engineering and regenerative medicine.
MATERIALS AND METHODS
Preparation of gelatin hydrogels
The method of hydrogel preparation is deeply described by Coester CJ et al (12). Briefly, 1,25 g of natural type A gelatin powder (from porcine skin) (Sigma-Aldrich Steinheim, Germany) was added to 25 ml of distilled water under heating. After 10 min of stirring, 500 ml of three different concentration of GA (2,5%, 5% and 8%) (Sigma-Aldrich, Steinheim, Germany) was added to crosslink the gelatin. Each group was done in triplicate. The solution was rapidly homogenized and dropped in a petri dish. Jellification process was performed at room temperature during10 minutes.
Characterization of macroscopic properties
Once biomaterials gelled, a macroscopic evaluation was performed to test the homogeneity, color and transparency of the gelatin. Moreover, a thermal test was performed to determine its stability at physiological temperature incubating the hydrogel at 37ºC for 24h.
Characterization of microscopic properties
Histological evaluation
For the histological analyses a representative sample was harvested of each hydrogel. The fragments were fixed in 10% buffered formalin for 24h at room temperature. Fixed samples were routinely dehydrated and embedded in paraffin, and transverse 5 μm sections were obtained from their central parts. All samples sections were stained with hematoxylin and eosin (HE) and observed and captured using light microscopy at 200X (Nikon eclipse 90i, Japan).
Structural and ultrastructural evaluation
For the structural and ultrastructural evaluation, the samples were fixed in 2.5% GA in 0.05 M cacodylate buffer, pH 7.2, at 4°C for 6 h. Then the samples were washed three times in 0.05 M cacodylate buffer, pH 7.2, at 4°C and randomly assigned to scanning electron microscopy (SEM) samples were dehydrated in increasing concentrations of acetone (30–100%), subjected to the critical point method and covered with gold. Samples were transversally mounted in stabs. Analyses and imaging were carried out using a FEI Quanta 200 environmental scanning electron microscope (FEI Europe, Eindhoven, The Netherlands).
RESULTS
Preparation of the hydrogels
The application of the previously described methods resulted in the gelation of all replicates in each group obtaining solid gelatin hydrogels. Gelation of biomaterials was GA-dependent as higher concentrations required shorter periods of gelation. However, in no cases gelation longed more than 30 min.
Macroscopy characterization of crosslinked type A gelatin
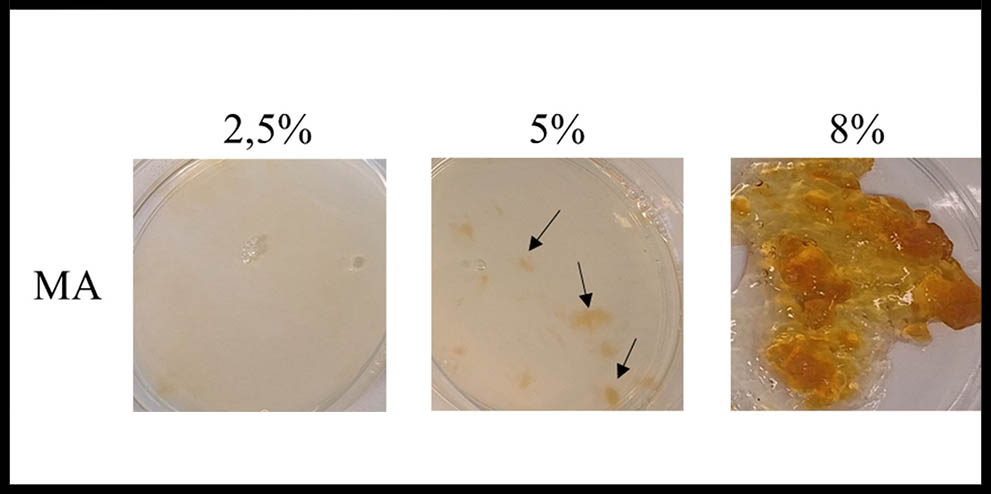
2,5% GA concentration hydrogel presented a homogenous appearance according with its particular transparency and a light clear color. Although it presents a gel state at room temperature, its thermal stability was compromised when the temperature rises physiological conditions and it melts at 37ºC. Gelatin crosslinked with 5% GA exhibited some distortions in its homogeneity emerging some areas with different crosslinked properties (see arrows in Figure 1). Moreover, transparency was maintained through the homogeneous areas but not in the more crosslinked ones which correlate with the areas of a more intense yellow color. Furthermore, gel state was maintained in the physiological range of temperatures exhibiting a thermostable characteristic. On the contrary to the above mentioned 8% GA concentration hydrogel, showed a complete heterogeneous appearance with non-defined areas of different crosslinked properties. This hydrogel did not spread over the dish and its color turned faint yellow compromising the transparency of the gel. Finally, the thermal stability was confirmed as no differences were found between room temperature and 37ºC.
Figure 1: Macroscopic analysis of crossliked gelatin hydrogels. Arrows show areas with different crosslinked properties.
Microscopic characterization of crosslinked type A gelatin
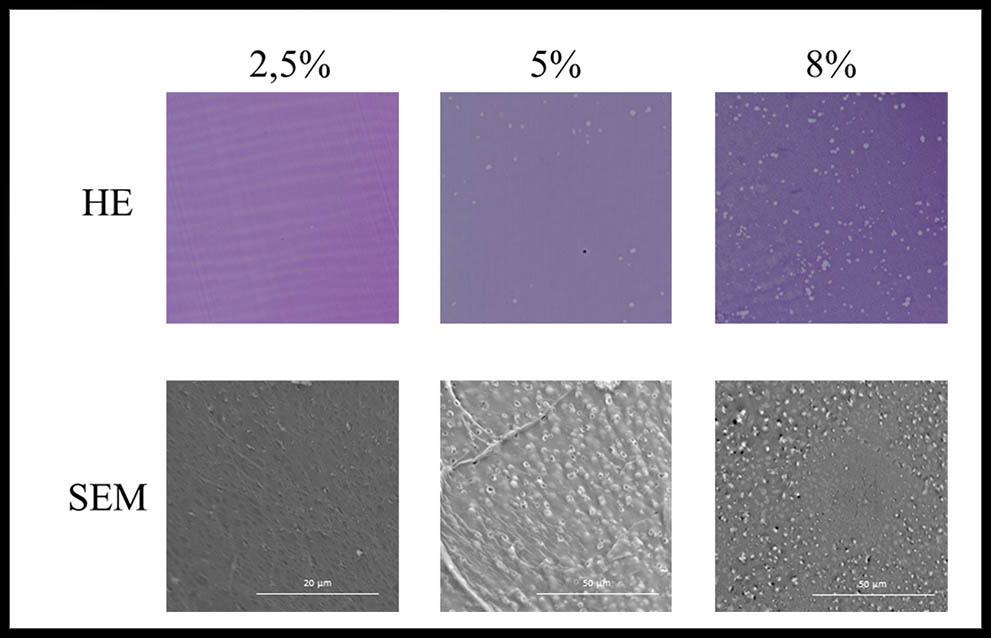
The Hematoxylin-eosin staining revealed a majority of basophil structures in all the experimental groups and different structural patterns appeared according to the crosslinking degree of the hydrogel as shown in Figure 2. An organized structure with fibers aligned in parallel were only found in 2,5% GA crosslinked hydrogels whereas a typical porous pattern was found in the other experimental groups (5% and 8%). Nevertheless, the hydrogel crosslinked with 8% GA manifested a higher degree of porosity with a more defined porous structure along the transversal section of the sample. Apart from this, scanning electron microscopy analysis confirmed that Type A gelatin ultrastructure have a greater number of smaller and more defined pores in high glutaraldehyde concentrations compared with 2,5% GA crosslinked hydrogels.
Figure 2: Microscopic analysis of crosslinked gelatin hydrogels. HE: Hematoxylin-eosin staining 200X. SEM: scanning electron microscopy.
DISCUSSION
Biomaterials play a key role in tissue engineering as they provide the scaffold where cells should carry out different biological processes as migration, differentiation or proliferation. In this sense, natural materials as gelatins are biologically active molecules and typically promote excellent cell adhesion and growth which are necessary for the development of artificial tissues and organs (3).
Despite the numerous advantages above commented of gelatin hydrogels show a lack of mechanical properties and a crosslink step is vital to enhance them in order to obtain scaffolds reassembling the physical characteristics of native tissues (13). In this study different concentrations of GA used as a crosslinker agent have been tested to generate different final biomaterials with different properties to broaden the potential applications of gelatin hydrogels to tissue engineering
According to the macroscopic evaluation, transparency is maintained in the less crosslinked hydrogel which is an essential property of cornea (14). Its highly organized collagen lamellae could provide mechanical support and biophysical properties required for transparency. Results shown in the HE staining strengthen the idea of a linear pattern in the structural evaluation. These results may suggest that low GA concentration may allow the development of homogeneous, transparent and highly organized biomaterials, especially interesting for cornea regeneration. Nonetheless, 2,5% GA concentration does not crosslink enough collagen molecules to ensure thermal stability at physiological temperature. Consequently, more studies are needed in order to improve thermostability while maintaining transparency.
5% and 8% GA crosslinked gelatin hydrogels exhibited a porous pattern in both HE staining and scanning electron microscopy analyses. More defined porous were found in the 8% GA crosslinked hydrogel. Besides high GA concentration is related to a lack of malleability and its rapid crosslinking reaction (90-120 s) makes it an unsuitable substitute. On the other hand, 5% GA crosslinked gelatin hydrogel showed a porous pattern with a more homogeneous macroscopic appearance. According to these results, the high porosity of this biomaterial could facilitate diffusion and perfusion of oxygen in bioengineered tissues. In addition, porous pattern obtained in 5% GA crosslinked gelatin hydrogels could meet the specific requirement of cardiovascular engineered tissues due to its interbranching 3D network that certainly could enhance the nutrients and oxygen diffusion, hemodynamic performance, cellular infiltration and organization (15, 16).
According to recent studies hydrogels are promising biomaterials to generate controlled properties of scaffolds through crosslinking processes. A crosslink is a physical or chemical bond that connects the functional groups of a polymer chain to another one through cova- lent bonding or supramolecular interactions such as ionic bonding, hydrogen bonding, etc (17). However, new substitutes should be generated applying different crosslinker agents such as genipin, carbodiimide or citric acid (18, 19) to improve its biophysical properties, thermostability and histological characteristics that resembles native tissues in order to build up a more translational approach in Tissue Enginering.
CONCLUSSION
In this study gelatin potential for tissue engineering has been tested. Optimal conditions were only found in 5% GA crosslinked hydrogels. Still, interesting findings were obtained that could be helpful to design controlled-properties scaffolds regarding its crosslinked degree that would facilitate the production of more suitable tissue-like products.
REFERENCES
- Nerem RM, Sambanis A. Tissue engineering: from biology to biological substitutes. Tissue Eng. 1995;1(1):3-13.
- O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Materials Today. 2011;14(3):88-95.
- Keane TJ, Badylak SF. Biomaterials for tissue engineering applications. Semin Pediatr Surg. 2014;23(3):112-8.
- Lu L, Peter SJ, Lyman MD, Lai HL, Leite SM, Tamada JA, et al. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21(18):1837-45.
- Liu D, Nikoo M, Boran G, Zhou P, Regenstein JM. Collagen and gelatin. Annu Rev Food Sci Technol. 2015;6:527-57.
- Sajkiewicz P, Kolbuk D. Electrospinning of gelatin for tissue engineering–molecular conformation as one of the overlooked problems. J Biomater Sci Polym Ed. 2014;25(18):2009-22.
- Zhan J, Lang P. The Review on Electrospun Gelatin Fiber Scaffold. Journal of Research Updates in Polymer Science. 2012;1(2):59-71.
- Butcher AL, Offeddu GS, Oyen ML. Nanofibrous hydrogel composites as mechanically robust tissue engineering scaffolds. Trends Biotechnol. 2014;32(11):564-70.
- Jiang Q, Reddy N, Yang Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010;6(10):4042-51.
- Martinez AW, Caves JM, Ravi S, Li W, Chaikof EL. Effects of crosslinking on the mechanical properties, drug release and cytocompatibility of protein polymers. Acta Biomater. 2014;10(1):26-33.
- Reddy N, Reddy R, Jiang Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015;33(6):362-9.
- Coester CJ, Langer K, van Briesen H, Kreuter J. Gelatin nanoparticles by two step desolvation–a new preparation method, surface modifications and cell uptake. J Microencapsul. 2000;17(2):187-93.
- Karimi A, Navidbakhsh M. Material properties in unconfined compression of gelatin hydrogel for skin tissue engineering applications. Biomed Tech (Berl). 2014;59(6):479-86.
- Ghezzi CE, Rnjak-Kovacina J, Kaplan DL. Corneal tissue engineering: recent advances and future perspectives. Tissue Eng Part B Rev. 2015;21(3):278-87.
- Fioretta ES, von Boehmer L, Motta SE, Lintas V, Hoerstrup SP, Emmert MY. Cardiovascular tissue engineering: From basic science to clinical application. Exp Gerontol. 2018.
- Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res. 2014;114(2):354-67.
- Daemi H, Rajabi-Zeleti S, Sardon H, Barikani M, Khademhosseini A, Baharvand H. A robust super-tough biodegradable elastomer engineered by supramolecular ionic interactions. Biomaterials. 2016;84:54-63.
- Mallick SP, Sagiri SS, Singh VK, Behera B, Thirugnanam A, Pradhan DK, et al. Genipin-Crosslinked Gelatin-Based Emulgels: an Insight into the Thermal, Mechanical, and Electrical Studies. AAPS PharmSciTech. 2015;16(6):1254-62.
- Oryan A, Kamali A, Moshiri A, Baharvand H, Daemi H. Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol. 2018;107(Pt A):678-88.
ARTICLE INFORMATION
Corresponding Author: Campos F. Departamento de Histología – Facultad de Medicina – Universidad de Granada