68
José Manuel Muñoz Olmedo
Basics of isolation and cultivation of chondrocytes
that chondrocyte phenotype is not stable
in vitro
, in particular
in monolayer culture (12). Most data also suggest that the
major phenotypic alterations are initially observed in the
superficial zone of early-stage osteoarthritic cartilage, where
chondrocytes express de novo abnormal, non-chondrocytic
genes; in particular, they express the enzymes required for
degrading the matrix that surrounds the cells as well as many
of the cytokines and growth factors relevant for turning on
the catabolic processes within cartilage (13).
Immunophenotype characterization of chondrocytes
was performed by flow cytometry, which offers the possibility
to assess and quantify a large number of epitopes on single
cells within a short period of time. Imunophenotypic analysis
of cells isolated from solid tissues through enzymatic
digestions might be compromised due to the reduction or
even total loss of surface molecules sensitive to enzymatic
treatment. Collagenase type II used in our study did not
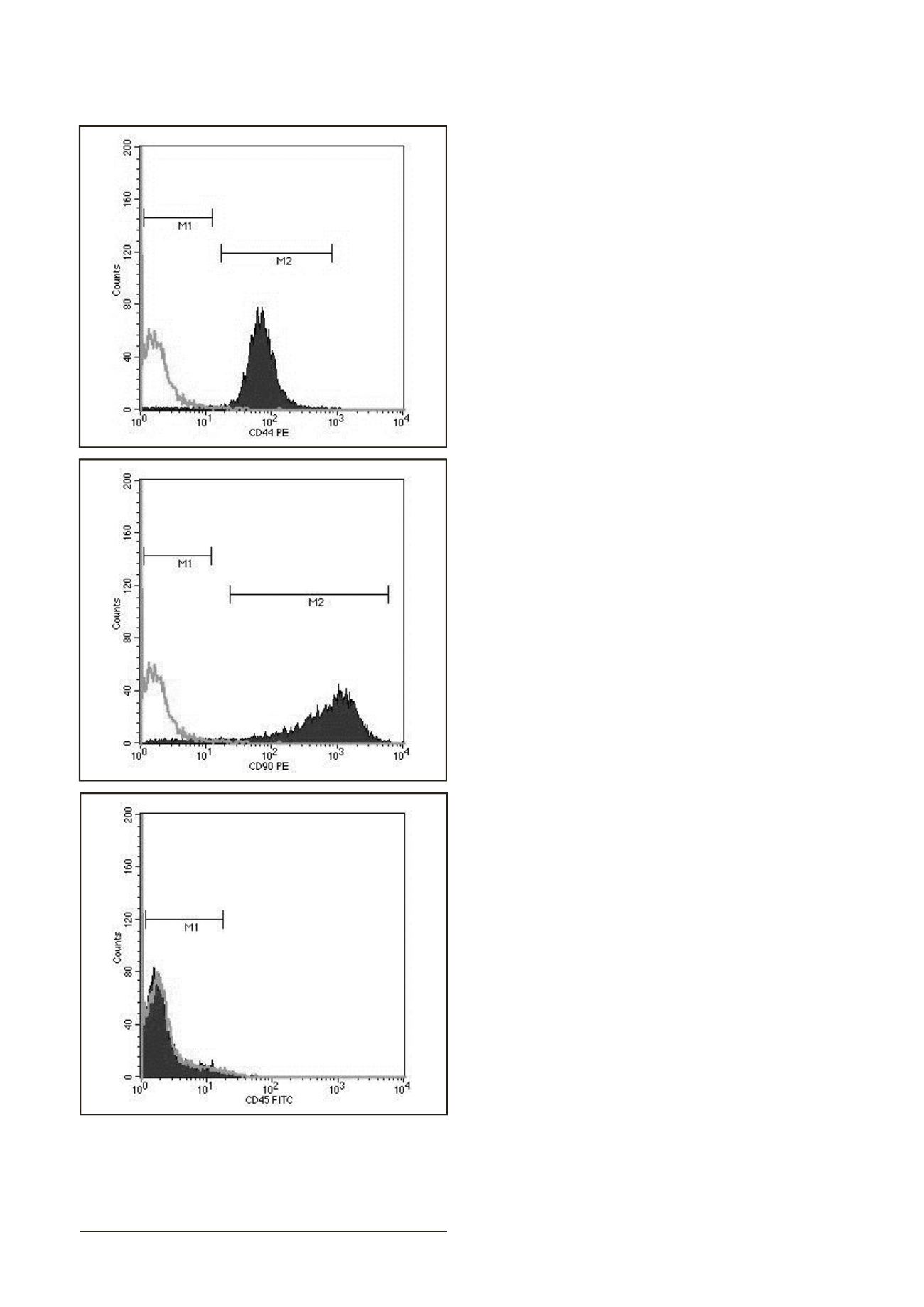
impair the detection of the CD90, CD44 and CD45 markers. We
have confirmed expression of the hyaluronan receptor CD44
and CD 90 (Thy-1) on chondrocytes (1P) as was previously
reported (14, 15, 16). The hyaluronan receptor CD44 belongs
to the polymorphic family of CD44 glycoproteins, which have
been implicated in a variety of cellular functions including
adhesion to hyaluronan and collagen, and which is present
in normal chondrocytes (15). It was previously reported
that CD90 is also expressed in a minority of chondrocytes
in normal articular cartilage (16). Up-regulation of markers
on chondrocytes regarded as distinctive for mesenchymal
stem cells (CD90 among others) during monolayer culture
suggested that dedifferentiation leads to reversion to a
primitive phenotype (17). Hematopoietic marker CD45
known as the leukocyte common antigen was not expressed
on chondrocytes. CD 45 should not be present in normal
chondrocytes but can appear in dedifferenciated ones (15).
It is important to enhance that cells used in this study
were not applied in clinical practice and basic characterization
of chondrocytes was the only aim. Nevertheless, weakness
of this study is that we did not include a control of
healthy articular cartilage and from younger patients with
osteoarthritis due to the limitation of donors. In spite of,
some investigators reported irreversible changes in phenotype
between chondrocytes isolated from OA cartilage versus those
of young healthy joints (18, 19, 20). Other reports indicate
comparable proliferation or differentiation potential of OA
chondrocytes (21, 22).
CONCLUSION
Basics methods of
in vitro
isolation and cultivation of
chondrocytes from OA cartilage were realized in this work.
The present study contains also some limitations. More
precise characterization of surface and intracellular markers of
chondrocytes and comparison with a control group of young
healthy patients should be done. In conclusion, we can assume
that human articular chondrocytes obtained from elderly patients
with osteoarthritis (stage 3) maintained a chondrocyte phenotype
and could be potentially used for autologous implantation. We
have standardized the conditions for cultivation to minimize the
risk of
in vitro
cell contamination according to GLP standards
since
chondrocyte manipulation for autologous
implantation
requires standardised protocols ensuring that the cell product is
therapeutically effective and safe.
ACKNOWLEDGMENT
This work was done during Research exchange project
organized by the International Federation of Medical Students’
Associations (IFMSA) and supervised by Denisa Harvanová and
Tímea Špaková, both from Associated Tissue Bank of Faculty
Figure 3. Flow cytometric analysis of chondrocytes (P1). Cells were
positive for CD44, CD90 and negative for CD 45.